Welcome to Changzhou Unique Medical Co.,Ltd!
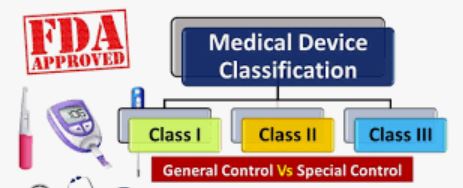
A Class I medical device are those devices that have a low to moderate risk to the patient and/or user. Today, 47% of medical devices fall under this category and 95% of these are exempt from the regulatory process. If a device falls into a generic category of exempted Class I devices, a premarket notification application and FDA clearance is not required before marketing the device in the U.S. However, the manufacturer is required to register their establishment and list their generic product with FDA. Examples include enema kits, elastic bandages, manual stethoscopes, and bedpans.